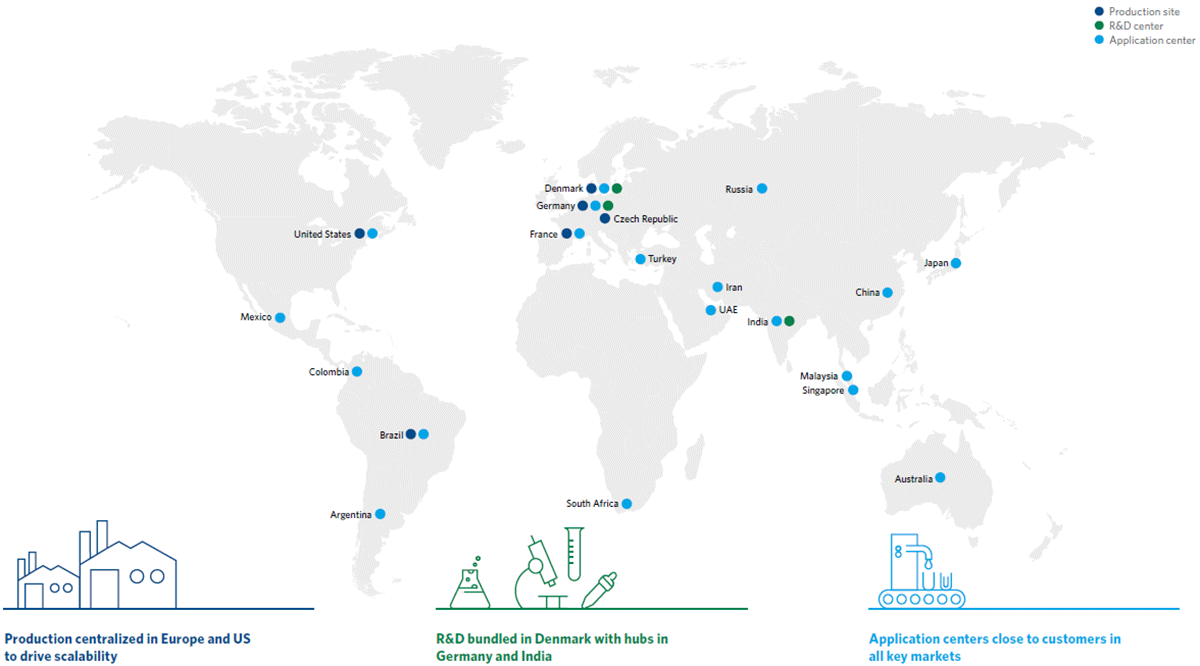
At Chr. Hansen Human Health, we are committed to ensuring the highest possible quality for our probiotics. Our probiotic products are manufactured in facilities specifically designed for the stability and safety of probiotics.
Our cutting-edge facilities reflect our commitment to quality. We have invested in innovative buildings, equipment and technologies that enable us to deliver high-quality products, raising the bar for what is possible within our industry.
We offer a wide range of custom services depending on your needs, and our facilities are well-equipped to facilitate all processes related to production, from probiotic fermentation and freeze-drying to formulation, encapsulation, bottling and packaging. This fully integrated production process makes us one of the few probiotic manufacturing companies worldwide with end-to-end production, giving us greater flexibility and oversight than would otherwise be possible.
Fermentation facilities – Copenhagen and Roskilde in Denmark; Madison, Wisconsin in USA
We operate two state-of-the-art production sites – one each in Denmark and the United States – where the probiotic fermentation process occurs. Our sites are designed to maximize yields while maintaining ideal conditions for probiotic stability and functionality. These facilities help to set the industry standard for excellence and support the production and protection of unique, top-quality probiotic strains.
Between our sites we can produce probiotics at three different quality levels: dietary supplement grade, pharmaceutical grade and infant grade (suitable even for preterm infants). With quality checks throughout the process and in-house freeze dryer availability, our facilities enable comprehensive control of the probiotic fermentation process, ensuring quality, purity and safety in every batch.
Denmark Certifications: KOSHER, HALAL, ISO 14001:2004, FSSC 22000, including ISO 22000:2005, Authorization/GMP and HACCP Certification from the Danish Veterinary and Food Administration for the production of foods and dietary supplements, Manufacturing license from the Danish Health and Medicines Authority for the production of medicines, Successful FDA inspection.
USA Certifications: KOSHER, HALAL, NON-GMO Project Verified, FSSC22000.
Our finished product facilities
Our team of experts oversee the production process from start to finish. They formulate, flavor, manufacture, test and distribute our scientifically supported probiotic solutions. Once our probiotic strains have been produced, they can be deployed in a variety of dosage forms. We offer options such as blended powders, capsules, chewable tablets, lozenges, probiotic shots, probiotic oil drops (POD) and T-WIN™ stick. All of these dosage forms are created with a continued emphasis on viability throughout their shelf life. This is important because probiotics are unlike any other nutritional supplements, they are live microorganisms with unique requirements for survivability.
This communication is only intended for business-to-business and health care professionals. This communication is not intended for consumers of final consumer goods. Nothing on this page is meant to be perceived as an approved claim.